FAQs
How They Glow (Fluoresce) - The Physics
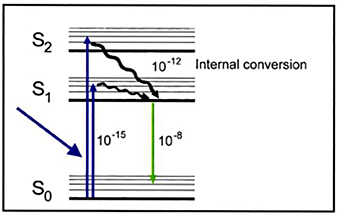 Jablonski Diagram
Jablonski Diagram
A Jablonski diagram, a schematic typically used to illustrate the electronic states of an ion in a crystal lattice and the transitions between them,
is a valuable tool for understanding the fluorescence process. In the Jablonski diagram, electronic (energy) states are indicated by bold horizontal lines.
The thin horizontal lines above them represent vibrational rotational sublevels. Electrons are normally at the lowest energy state, indicated by S0.
Photons—elemental particles of electromagnetic radiation, including light—are indicated by the blue arrow entering from the left.
When a photon with appropriate energy interacts with an ion, the photon may be absorbed, causing an electron to jump to an excited state (S1, or S2,
in the diagram) or one of its sublevels. By "appropriate energy," we mean energy corresponding to the difference between the ground state and any of the sublevels
in either of the excited states. Thus not all incident photons are equally likely to be absorbed. This transition process is very fast, on the order of 10-15 seconds.
An excited-state electron rapidly loses some of its energy to vibration (heat), a process called internal conversion, and falls to the lowest
level of the first excited state (S1). The time scale for this process is on the order of 10-12 seconds. From there the electron may fall to
one of the sublevels of the ground state (S0), emitting a photon with energy equivalent to the energy difference of the transition. This happens at a time
scale of nanoseconds after the initial photon was absorbed. Since the emitted photon has less energy than the absorbed photon, it is at a longer wavelength.
This explains the seemingly magical process of fluorescence that converts light of one wavelength to another, and transforms invisible UV light to the vivid examples of
mineral fluorescence that collectors so admire.
From Mazel, Charles, PhD, and Earl R. Verbeek, PhD. "Fluorescence of Minerals Under Blue Light."
Picking Table Vol. 55, No. 2 Sept. 2014: 13-25.
Fluorescence
Most commonly, fluorescence refers to the property of emitting visible light during radiation by ultraviolet light.
The visible light given off can be of almost any color, depending on the substance which is fluorescing and, to a lesser extent, on the wavelength
of the ultraviolet radiation that causes the fluorescence.
Phosphorescence
We have just discussed how visible fluorescent light is radiated by a fluorescent material while it is exposed to ultraviolet light.
Now let’s see what happens when the ultraviolet light is removed. With most fluorescent substances, the electrons settle back quickly
into their balanced orbits and there is no further radiation of visible light. But in some materials, the electrons are slow in returning
to their normal orbits. In this case, the atoms continue to give off light as long as the electrons are returning to their normal state.
This continued emission of light after the ultraviolet radiation has been removed is known as phosphorescence. Some materials will phosphoresce
for only a few seconds while others will continue to give off light (in ever diminishing intensity) for long periods.
Activators
Not all substances are fluorescent – in fact, most of them are not. In substances that do fluoresce, it has been found in most cases that
a small amount of some impurity must be present in order for fluorescence to occur. Few chemically pure minerals will fluoresce at all. But on the
other hand, the amount of the impurity is critical and if there is too much, the fluorescence will either be diminished or completely eliminated.
For example, the red fluorescent calcite from Franklin, New Jersey is activated by manganese in a quantity of about 3%. It has been found that manganese
content in the calcite of more than about 5% or less than about 1% will not permit fluorescence. The amount and type of impurity present determine the color
and intensity of the fluorescence. The mineral calcite seems to be particularly sensitive to impurity activation and specimens of calcite have been found that
fluoresce in practically every color. The amount of activator can be as important as the type.
What do I need to collect fluorescent minerals?
The following items are highly recommended for collecting fluorescent minerals:
• Sturdy boots, steel-toed work boots
• UV protection safety glasses
• Geologist’s hammer (not a claw hammer)
• Chisel
• Backpack and/or bucket
• A heavy dark cloth or black tarp
• Short wave and/or long wave ultraviolet lamp
Some common fluorescent minerals at Franklin and Sterling Hill
Ultraviolet (UV) light
Ultraviolet light is the portion of the electromagnetic spectrum from 400 nm to about 220 nm wavelength, which is not visible to the human eye.
It can be divided into three sub-bands, UVA (315–400 nm); UVB (280–315 nm); and UVC (180–280 nm).
Commercial ultraviolet lamps are commonly found in three different wavelengths, they use filter glass to help eliminate visible light emitted by the lamp:
UVA or long wave UV at around 360 nm causes many minerals to fluoresce, but other commonly fluorescent minerals may not respond. UVB or mid-wave
UV at around 312 nm causes some minerals to fluoresce, it can be a valuable tool for identification. UVC or short wave ultraviolet lamps at around 254 nm
wavelength are the most popular for mineral collectors, they cause many Franklin and Sterling Hill minerals to fluoresce brightly. UVC light is potentially harmful
to skin and eyes, this is the part of the electromagnetic spectrum that will give you a sunburn!
Note that inexpensive commercial “black lights” available at stores and gift shops have a wavelength of between 350 – 450 nm, and many fluorescent minerals
do not show a strong visible response to these lamps.
Places to collect fluorescent minerals
The Buckwheat Dump at Franklin Mineral Museum:
The Franklin Mineral Museum's mission is to preserve knowledge of the mineral wealth, geology, and history of what has been called
"the greatest mineral locality on earth." It is a nonprofit educational institution, located at 32 Evans Street, Franklin, Sussex County, New Jersey.
The museum was incorporated June 2, 1964.
Franklin, NJ and its close neighbor Ogdensburg are the location of the world's most famous zinc mines. The Franklin mine is especially famous
for mineral fluorescence, and for the variety of rare mineral species discovered there.
On September 13, 1968 the State of New Jersey passed a resolution declaring the Borough of Franklin: "the Fluorescent Mineral Capital of the World."
These zinc-iron-manganese deposits are unique in their mineral composition and have produced more than 360 mineral species. There were three major ore minerals
(zincite, franklinite, and willemite), all of which were unknown to science when they were found. Zincite was the first to be correctly analyzed, in 1810 by Archibald Bruce.
The Mine Run Dump at Sterling Hill Mining Museum:
Sterling Hill Mining Museum’s mission is to tell the story of the Sterling Hill Mine and to inspire lifelong learning about earth sciences,
engineering, and the responsible use of the Earth’s nonrenewable resources. It is located at 30 Plant St, Ogdensburg, Sussex County, New Jersey.
The Sterling Hill Mining Museum opened for tours on August 4, 1990 and is now visited by more than 40,000 people annually.
Visitors to the Sterling Hill Mining Museum can discover a piece of New Jersey history as they enter the grounds of this former industrial site.
The museum's facilities, including exhibits, are housed in the original buildings used by the New Jersey Zinc Company when the mine was operational.
The Sterling Hill Mining Museum is the only place in New Jersey where members of the public can tour an underground mine and see for themselves
how mining was done at one of the world's most famous mineral localities.
